What is the difference between a Covid-19 rapid Point-of-Care test and a self-test?
A health practitioner or qualified employees working under their supervision can perform point-of-care diagnostics to screen a client for COVID-19 outside the laboratory environment. This guarantees that a suitable health practitioner, or a qualified individual working under their supervision, is accessible to ensure an adequate sample is obtained, the results are correctly interpreted, and urgent clinical advice and treatment are provided if necessary.
COVID-19 point-of-care serology testing
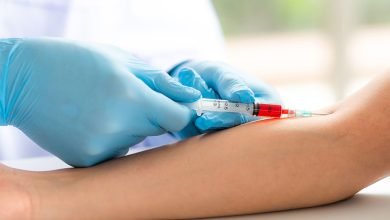
Point-of-care COVID-19 serology assays reveal antibodies produced by humans in the days following infection with SARS-CoV-2. These tests, like pregnancy tests, are often delivered in a compact plastic cartridge and require blood samples for testing. Antibody serology tests often provide information regarding virus exposure in the past.
Rapid antigen test for COVID-19 at the point of care
Rapid antigen point-of-care tests detect the presence of SARS-CoV-2 virus protein. While quick antigen testing can produce results in 15-30 minutes, they are generally believed to be less sensitive than polymerase chain reaction (PCR) tests, which remain the gold standard for SARS-CoV-2 diagnosis. Positive results must be confirmed using a PCR test.
What are rapid point-of-care COVID-19 tests?
Rapid point-of-care tests are intended to confirm or rule out COVID-19 infection in individuals with or without signs of COVID-19 infection.
They are portable, allowing them to be employed at the point of care regardless of the location of the patient;
– are simple to conduct, requiring little additional equipment or complicated preparation requirements;
– are less costly than conventional laboratory tests;
– do not necessitate the use of a specialized operator or configuration; and
– deliver outcomes while you wait.
We were interested in two types of fast point-of-care commercially available tests: antigen and molecular assays. Antigen tests look for proteins on viruses and are available in disposable plastic cassettes similar to pregnancy tests. Rapid molecular tests identify the virus’s genetic material in the same manner that laboratory methods do but utilize smaller instruments that are easier to travel and set up outside of a specialized laboratory. Both nasal and throat samples are required for testing.
Why is this a critical question?
Individuals with suspected COVID-19 infection require rapid confirmation of disease to self-isolate, obtain treatment, and notify close contacts. COVID-19 infection is now confirmed by a laboratory test called RT-PCR. Which requires specialized equipment and frequently takes at least 24 hours to complete.
Rapid point-of-care tests have the potential to expand access to testing for a large number of people, both with and without symptoms and in contexts other than healthcare facilities. Faster diagnosis, if accurate, may enable people to take appropriate action more immediately, perhaps reducing the spread of COVID-19.
What were we hoping to discover?
The purpose of this study was to determine whether commercially available fast point-of-care antigen and molecular tests are accurate enough to detect COVID-19 infection reliably and to determine whether accuracy varies between patients with and without symptoms.
What did we accomplish?
We sought studies that assessed the accuracy of any commercially available fast antigen or molecular point-of-care test in individuals tested for COVID-19 using RT-PCR. Individuals may be tested at a hospital or in the community. Individuals with or without symptoms may be tested in studies.
Tests have to be performed with minimal equipment, safely and without risk of infection from the sample, and findings available within two hours of sample collection.
What we discovered
The review comprised 64 studies. They examined a total of 24,087 nose or throat samples and confirmed the presence of COVID-19 in 7415 of these samples. The studies examined sixteen distinct antigen testing and five distinct molecular assays. They primarily occurred throughout Europe and North America.
As the highly contagious Omicron variety continues to spread across the country, many people—even those entirely vaccinated—are beginning to wonder if the development of cold and flu symptoms is a true sign of COVID-19 infection.
However, COVID testing has developed into a complex subject. As Omicron’s popularity grew, scheduling visits at locations where laboratory process results became increasingly challenging. As a result, many individuals turned to self-administered COVID-19 examinations. Often referred to as fast tests, these kits are available in pharmacies and online, allowing anyone to test themselves — and receive results — in the privacy of their own home in a matter of minutes.
Regrettably, at-home examinations have become increasingly challenging to locate, if not impossible. The federal government intends to reduce the bottleneck by compelling private insurance companies to cover at-home tests and provide Americans with 1 billion free quick tests.
If you can obtain a test, you may find the various possibilities confusing. In which circumstances is a laboratory-based (commonly referred to as PCR) test preferable? Which one should you acquire if you’re traveling and need to demonstrate a negative COVID test? Are all of them equally accurate? Are some more productive than others? And how far up your nose does each type of Q-tip go?
While some of these questions are straightforward, others are more challenging, particularly accuracy. That is because all of the tests — and there are hundreds of them from an increasing number of companies and laboratories — are available only with an emergency use authorization from the Food and Drug Administration (FDA) (EUA). As a result, they have not been as carefully evaluated or scrutinized as other FDA-approved medical tests.
Because the virus is new, all tests are unique. Which means we lack a long track record comparing results and a real gold-standard test.
Additionally, with each new version, additional issues occur. Recently, there has been debate over whether throat swabs or saliva samples are more efficient in detecting Omicron than nasal swabs—or whether quick tests are less effective at detecting Omicron.
What method is used to get a sample?
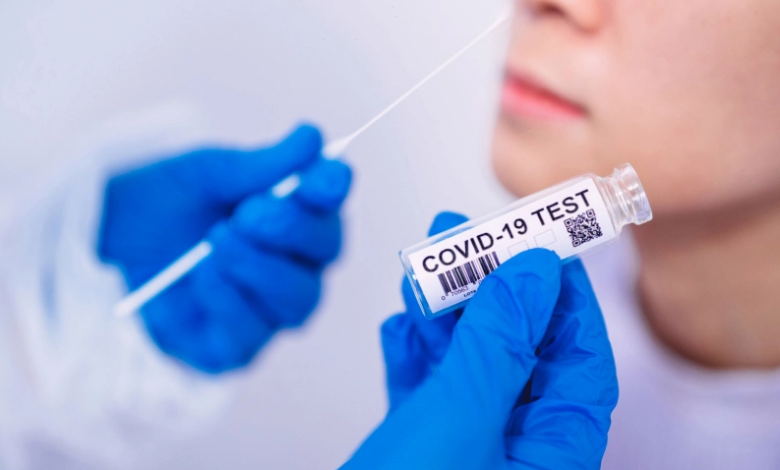
Usually, with the use of a swab put into the nostrils. There are three distinct procedures for collecting nasal specimens:
Nasopharyngeal: A health care expert inserts a long swab deeply into your nostril to collect fluid from the back of your nose.
Mid-turbinate: This approach, which a professional can teach or perform, entails inserting a soft swab into the nostril (less than one inch) to obtain a sample.
Anterior nasal swab: This test, which can be self-administered with the assistance of a qualified health care provider or performed by a health care professional, includes inserting a swab three-quarter of an inch into the nostril and twirling it around at least four times to obtain a sample.
How accurate are COVID-19 fast tests?
Rapid COVID-19 testing frequently offers results within minutes and does not require professional laboratory analysis.
Most fast tests are antigen tests, and the names are frequently used interchangeably. However, the CDCTrusted Source discontinued the term “quick” when referring to antigen tests. As the FDA has approved laboratory-based antigen tests as well.
Rapid testing, often known as point-of-care tests, can be performed at the following locations:
a residence with a residence COVID-19 testing is available in physician’s offices, pharmacies, school clinics, and long-term care facilities.
Airports are ideal locations for drive-through testing.
You or a medical practitioner will insert a cotton swab into your nose, throat, or both to collect mucus and cells during the test. Typically, your sample is transferred to a strip that changes color if you test positive for COVID-19.
While these tests are quick. They are not as accurate as laboratory testing since they require a higher concentration of virus in your sample to give a positive result. Rapid testing has a significant likelihood of producing a false negative effect.
A false negative result indicates that you do not have COVID-19 when you do.
Read More: Relieving Back Pain: Simple and Effective Treatment Options